Home Creme de Vape Blog Posts on topic: diacetyl
Creme de Vape Blog
Shortfills have arrived
Well, finally, shortfill "shake and vape" e-liquids have arrived at Crème de Vape. They provide a cost-effective way for vapers to obtain larger bottles of e-liquid and avoid having to deal with all those little bottles.
Why has it taken so long for us to get them in?
Good question - I'll need to provide a bit of background to answer it.
We noticed shortly after the end of the TPD transition period in May 2017, retailers started offering large bottles of flavoured e-liquid without nicotine, with empty space for TPD compliant flavourless nicotine liquid to be added by the consumer because the TPD/TRPR regulations do not currently apply to liquids without nicotine. This has been seen by many as a positive development, however, it was unfortunate that so many producers rushed in to take advantage of this loophole with all manner of completely untested liquids.
We've spoken to many companies over the years who simply don’t care to test their product and seem not to be bothered at all about what their customers are ingesting. I can’t count the number of times that our request for test results has elicited a response along the lines of “no-one else has asked” or “you're the only one with a problem”. These new untested shortfills took us back to how the e-liquid industry used to be pre-TPD when we faced extreme difficulty getting any kind of information about product content from manufacturers, and we had to run our own testing because we refuse to stock potentially unsafe liquids.
The industry had been aware of the problem since Dr Farsalinos' 2014 report where he revealed he'd tested and found potentially harmful ingredients in a lot of e-liquids on the market. He tested for Diacetyl (2,3-Butanedione) and Acetyl Propionyl (2,3-Pentanedione) which are buttery/creamy flavoured chemicals which have been linked to permanent lung damage when inhaled. See his study report here. Consumers and manufacturers were starting to sit up and take notice.
We put our cards on the table some years ago (when we were known as Cloud9Vaping) when we publicly blogged about our long-standing e-liquid testing policy (see our blog post here). We stated we'd removed a range of e-liquid from sale shortly after commencing stocking it, because our testing revealed that contrary to the manufacturer's own assertions, the liquids contained very high levels of these potentially harmful ingredients. Many customers asked us which range we had removed from sale, so eventually we revealed the full story which lead to a huge debacle much of which was publicly played out. The manufacturer then threatened us with legal action for revealing information that they already knew about, but had concealed from retailers and consumers.
Gradually, the industry started to listen and vaping trade and consumer associations also started to recommend avoidance of liquids with these ingredients. Manufacturers started to actually recognise the problem and many began to take steps to deal with it and reformulate their liquids.
When the TPD/TRPR arrived, it mandated emissions testing and full ingredient disclosure, along with toxicology reporting for every single ingredient present. It also completely disallowed certain ingredients including the two I’ve mentioned above, which was a great step forward even though many of the other measures in the TPD have been ill-advised. See the TPD UK ingredient guidance. We thought it would finally rid the market of all poor quality liquids including those made with inappropriate ingredients that shouldn’t be inhaled – however that has not been the case as many producers of zero nicotine liquids have made no effort to perform any testing or comply with the banned ingredient list
The four ranges of shortfills we now stock have all been tested in exactly the same way as TPD compliant e-liquids, and none of them contain any of ingredients banned by the TPD, with the exception of Blackberry Crumble by Dinner Lady which contains a trace amount of naturally occurring (unavoidable) Diacetyl which is below the AFNOR recommended maximum level.
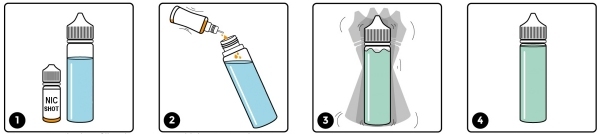
It’s possible that the unregulated shortfill market will be shut down at some point in the future when the regulators take notice and close this loophole. We expect the authorities to mandate that they be tested in exactly the same way as any other e-liquid and we would welcome this change. We only have to hope that they don’t put the 10ml limit on these liquids as well – if they do, it will be entirely because of those who tried to avoid testing and are happy to supply unknown substances for unsuspecting consumers to ingest.
Useful links
Tobacco and Related Products Regulations (TRPR)
TPD UK Ingredient Guidance
Liquid test results - update
The original content of the post, dated 28th June 2015 and entitled Liquid test results, was a table of test results of liquids supplied by Five Pawns (as well as other suppliers) and an explanation of those test results.
They declined to provide their own test results when we commenced trading at the start of 2015, accordingly (and in accordance with our product testing policy) we submitted 8 Five Pawns liquids to testing at an independent UKAS accredited laboratory.
We published the results approximately 7 weeks after providing them privately to Five Pawns.
Five Pawns took exception to the post, sent a cease and desist letter through their attorneys, threatening legal action if our post was not removed.
Whilst we stand by these results, we decided to take down the information to comply with Five Pawns’ request.
Our own solicitors have responded in robust terms rejecting allegations made by Five Pawns, and they have now released their own test results, and it is incontrovertible that liquid produced by Five Pawns contains the compounds in question.
Liquid test results
Edited: 29/06/15
The information relating to specific products withdrawn from sale as part of our testing/due diligence processes has been removed pending legal advice.
Update: 02/07/2015
Liquid test results - update
Update: 02/07/2015
Latest test results on our own brand liquid are now available.
Our liquid testing protocol
There's been a lot of discussion and debate about dangerous compounds that have been found in many flavoured e-liquids and food flavourings used in e-liquid production.
We felt it was about time to give a thorough explanation of exactly what we do, so that you can rest assured, we're taking every step we possibly can to provide as safe a product as possible, according to the current known risks.
We've been testing our products since 2011 at the same independant UK-based laboratory used by Trading Standards. The lab is accredited by the United Kingdom Accreditation service (UKAS) to the internationally recognised ISO 17025 standard which means they have demonstrated they are technically competant and have documented procedures and quality management systems in place to ensure they are producing precise, accurate and repeatable test data. This accreditation also means they are continually monitored and audited to ensure their procedures and methods continue to consistently provide accurate and valid results that can be trusted by governmental organisations and compared to test data produced by other accredited labs worldwide.
We don't generally publish our results alongside the liquids, because we anonymise the samples for company confidentiality. This "blind testing" policy is common in many industries, and was introduced to us by ECITA when we first began testing. This method wasn't an issue when we were simply running tests for our own internal due diligence. However, it has caused us to be unable to properly publish all of our own test results alongside the products on our site so we will be changing this policy in the future so we can publish all our test results with the product clearly identified.
Diethylene Glycol and Ethylene Glycol
Diethylene glycol (DEG) is a known contaminant that is often present in general reagent grade Glycerine that's not designed for ingestion or pharmaceutical use. Some research was released in 2009, claiming that the FDA sampled several pre-filled cartridges and found Diethylene Glycol (a poison) present. It's imperative that these compounds aren't contained in products designed for ingestion, although not much notice is given to this within our industry. We have historically tested all our liquids for both DEG and Ethylene Glycol to verify the purity of the diluents used in the product, and to-date, none of our pre-mixed e-liquids have ever tested positive for either of these compounds. We are proposing dropping this test from our protocol as we're confidentn our manufacturer's protocols eliminate all risks in this area.
Nicotine Assay
As nicotine can be dangerous in high doses, we feel it's prudent to test the actual nicotine level, in random e-liquid samples, both to confirm that it is within tolerance of the stated level, and to confirm it isn't present in liquids that are labelled zero nicotine. It has been our experience that some tobacco type flavour zero nicotine liquids can contain miniscule traces of nicotine, due to to the method of production involved in manufacturing some tobacco flavours, however, we have never seen any results above 0.04% nicotine (0.4mg/ml) in a zero nicotine e-liquid, and any product that tested above 0.02% (0.2mg/ml) would not be released for sale. The last time any of our zero liquids tested positive for traces of nicotine (at 0.01%) was in January 2013.
Propylene Glycol and Vegetable Glycerine Quantification
We run these tests primarily to ensure any product we advertise as "PG Free" is in fact completely free of PG, since these products will typically be chosen by consumers who have an allergy or an intolerance to Propylene Glycol. We also like to see the PG/VG ratios for our own consistency checks and so we can advise customers who have a particular PG/VG ratio preference.
Diketones
All flavourings used in our pre-mixed liquids, and available as concentrates for DIY mixing have been certified as safe for use in consumption via the digestive system - i.e. they are food-safe. However, this is not the whole story, since ingestion into the lungs via vapour is an entirely different pathway, and some flavourings that are completely safe to be eaten, are not safe to be vaped.
There are a family of compounds referred to as diketones, that pose some serious inhalation risks and should not be present in e-liquid. These compounds are used to provide a buttery/custard/vanilla type flavour and are currently found in many pre-mixed e- liquid ranges, particularly the more complex blends coming from the USA at the moment. The diketones of particular concern are Diacetyl (2,3-butanedione or DA) and 2,3-Pentanedione (Acetyl Propionyl or AP). We also test for the presence of Acetoin, which by itself, is not known to be harmful, but it can partially catalyse into DA under certain conditions, so we do test for acetoin, so that we are aware of the level, if present.
Diacetyl (DA) in its inhaled form, has been identified as the major cause of a serious lung condition observed amongst a group of popcorn factory workers exposed to large amounts of fumes containing DA in an industrial environment over an extended period of time. As yet, no tests have been performed on human inhalation via vaporisation of flavoured e-liquid, however, there is a published occupational safety limit of 66µg (66 parts per million), which means, if you vape 1ml of e-liquid, it must have less than 66µg or 0.0066% DA to be below the occupational safety limit. Our current testing is able to detect DA to 10 parts per million or 0.001%. None of our e-liquids or flavouings contain DA as a specific ingredient, and where we have identified it during testing, we have refused to stock the product or withdrawn it from sale if it was already on sale prior to receving DA-positive test results.
The dangers associated with inhaling DA have been known for some years, and we've been testing for the its presence since 2011, however, it was only in 2014 when Dr Farsalinos released the results of his research, that the industry realised flavouring manufacturers who were responding to the "noise" regarding Diacetyl, had been replacing it with Acetyl Propionyl (AP), and that AP has very similar inhalation risks and is not suitable for vaping either. Prior to this research, many vendors, including ourselves, were unaware that AP also posed a very real risk, and since this point, we have also included it in our testing protocol. The published occupational safety limit of AP is 135µg (135 parts per million), which means if you vape 1ml of e-liquid, it must have less than 135µg or 0.0135% AP in it to be below the occupational safety limit. Our current testing is able to detect AP to 10 parts per million or 0.001%.
As of the date of this article, we do not stock any pre-mixed e-liquids that have tested positive for DA or AP at any level.
We may take the view in future, that some consumers want these types of flavours, and we may decide to stock e-liquid that has tested positive for DA at a very low level, as it occurs naturally in some flavours. If we did this, we would impose a limit ourselves, of approximately 25 parts per million (0.0025%). If we stock any e-liquids in future that do contain traces of DA up to 25 parts per million (0.0025%) we will clearly indicate this information. Where our testing identifies an e-liquid contains DA above 25 parts per million (0.0025%), we will still continue to refuse to stock it. We are unlikely to alter our position on AP, since it never occurs naturally and is only present when it has been physically added.
Flavour Concentrates
We do also use the same testing protocol (excluding nicotine assay) on specific flavour concentrates where we believe, by virtue of their flavour profile, could contain harmful diketones. None of our concentrates have ever tested positive for Diacetyl, but some do contain AP in very low quantities. Where concentrated flavourings are concerned, assuming the concentrate is used at 10% and 5ml per day is vaped, our own self-imposed limit would translate to 250 parts per million (0.025%). We will not stock any new flavour concentrates that exceed this level, and those we currently stock that could exceed this level are clearly identified in our flavour concentrates product information download.
Further information
It should be noted that where a liquid has a buttery or custardy taste, but has tested free of DA or AP, the buttery note will have been achieved by Acetoin or Butyric acid, or a combination of these components. Butyric acid can cause irritation in some vapers and it can also be responsible for an unpleasant sour after-taste in certain concentrations or flavour combinations.
The amount of liquid you vape per day may exceed averages, thus exposing you to more risk, and indeed, if you are at all concerned about diketones, or other as-yet unidentified, potentially harmful component, we advise you steer clear of all buttery, custardy, vanilla, creamy & sweet flavours or consider vaping subtly flavoured, or even unflavoured liquids, rather than strongly flavoured recipes with many flavouring components present, in high concentrations.
Additionally, it should be remembered that vaping is a "reduced-harm" alternative to smoking - this doesn't mean it's completely harmless but that it's known to be much less harmful than smoking traditional combustible tobacco. The fact that liquids have tested free of these known risks, is not an indication that any one flavouring is more or less "safe" than any other - no long term or comprehensive studies have yet been performed on human inhalation of vaporised food flavourings.
Positive Action
In the recent past, we removed a well-known premium e-liquid range from sale because our test results indicated very high levels of DA (testing showed up to 100 ppm or 0.01%), and extremely high levels of AP (testing showed up to 2500 ppm or 0.25%). We trusted the manufacturer were doing their own due diligence but this experience was a reminder that there are no shortcuts. We are not prepared to take risks with your health and our company reputation, and will not stock any new liquid products until the supplier has provided comprehensive analysis performed by a known, accredited & independent laboratory, or we have performed our own analysis.