Home Creme de Vape Blog Posts on topic: MHRA
Creme de Vape Blog
TPD Update
We understand there is still some confusion about the EU Tobacco Products directive (TPD) which came into law across the EU on May 2016. The UK implementation is known as the Tobacco and Related Products Regulations 2016 (TRPR). It contains various restrictions and regulatory burden all of which will affect our customers to varying degrees. We’re currently in the transition period, and we can sell existing stock without restriction. This period ends on 19th May 2017, after which all products sold must meet the new requirements of the TRPR. Liquids without nicotine are exempt from these requirements.
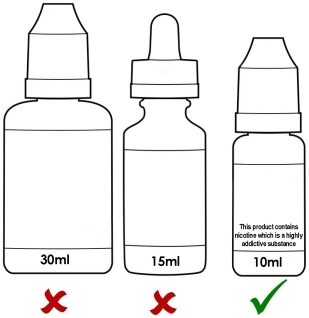
Maximum 10ml bottle size and maximum nicotine strength of 20mg/ml (2%).
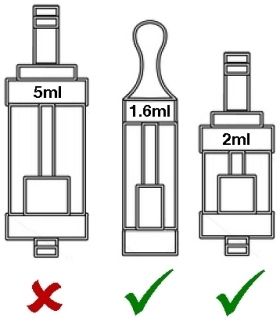
Maximum 2ml capacity for tanks, which must have a leak-free filling mechanism.
In addition to the size and strength restrictions, where a product contains nicotine, or could be used to contain nicotine the TRPR requires:
- A large warning about nicotine on the front and back of the packaging (even where the product is sold empty).
- Ingredients, composition and additional information and warnings to be on the outside of packaging and on enclosed leaflets.
- Products to be comprehensively tested (including their emissions during use) and the data notified to the governing body 6 months before products are placed on sale (or before November 20th 2016 for products already on the market).
- Sales of each notified product reported annually.
Some of the products you rely on may change to a compliant format, but many will not, and will become unavailable for sale to consumers in the UK and EU. The substantial costs involved in the testing and notification will lead to a further reduction in the variety of products on the market as most manufacturers will only be able to afford to test and submit their best sellers. Prices may increase slightly to cover the costs of the testing and notification, as well as the additional packaging.
We have been unable to import any non-compliant products since November 20th 2016, and so non-compliant products are beginning to sell out, and won’t be replenished. As you're browsing the website, you may notice a red box with the text "NOT PURCHASABLE IN THE EU AFTER MAY 19 This is your cue to buy now, whilst you still can, because you will not be able to purchase this product after this date. If you see this on a 30ml e-liquid, we recommend you browse the site to see if there is a 10ml compliant version available.
We advise you to ensure you have stocks of your favourite products on hand well before May and transition to compliant products as soon as they become available. We have been concentrating our efforts and working hard with our manufacturers to prepare compliant items over the last 6 months and although there are huge challenges to overcome, we are hopeful about the future of vaping post-2017.
For further information please see our earlier TPD blog post.
The future of vaping changes in May 2016
You may have heard rumours about upcoming regulations which will affect the availability of many of the vaping products you currently enjoy.
We’ve been hesitant to communicate much of this information directly to our customers thus far, because the situation has not been clear enough to be able to properly inform you about exactly what will happen. Information directly from UK regulators has slowly started to come through but there’s still grey areas and ambiguity in the information released so far, so it’s still quite difficult for companies to plan properly or advise their customers on what the future holds.
Having said that, we know a large proportion of our customers still don’t know anything about these looming changes and we felt it was time to give you a run-down of what we know so far as well as comment on what we don’t yet know. Apologies that this is quite a long post, but it’s a complex topic, and requires as much explanation as we can provide to ensure you are as well-informed as you possibly can be.
On May 20th 2016 The EU Directive 2014/40/EU1 also known as The Tobacco Products Directive (TPD) comes into force. This Directive changes how tobacco and related products are manufactured, marketed and sold within the EU and contains some very restrictive measures relating to vaping products2.
Many feel (us included) that the restrictions relating to electronic cigarettes didn’t belong within a directive relating to tobacco products at all, and that regulatory provisions for these innovative, and life-changing products belong in separate, more carefully considered legislation, which could have ensured vapers continued to have access to the products they need and the right to choose a much safer alternative to smoking. However, the directive as it is was pushed through to further regulate cigarettes, tobacco products, and vaping products, all in one go.
As a result of this legislation, many of the products we know and love will disappear and the products currently available on the market will become very limited. The rate of innovation will also slow due to the financial burdens and delay involved in bringing new products to market, compounded by the 6 month delay that’s been imposed for newly notified products. The regulations do however allow a sell-through period3 for the sale of old stock until May 2017.
Unfortunately, there’s still a LOT up in the air due to the regulators’ failure to unambiguously define what’s included and what isn’t as well as the fact they have repeatedly contradicted themselves on various documents we’ve seen. This uncertainty means that much misinformation is out there, and manufacturers and retailers are not yet able to state exactly what the position will be, even though the restrictions will take effect in just a few months’ time.
We’ve chosen to focus here on those elements of the legislation that will be most restrictive for existing vapers, however there are various other provisions that will dramatically affect vaping. One that is of great concern to those of us who are keen to spread the message of vaping to as many smokers as possible is the ban on all forms of advertising and promotional activity, which may include online discussion forums and specialist vaping broadcast channels as well as traditional print and TV/radio media. Please rest assured that we’re doing our absolute best to navigate our way through the legislation, and working with our manufacturers to ensure they are also up to speed, and capable of producing compliant products. However, these changes ARE coming, and all current and future vapers will be radically affected.
Read on for detailed information on some of the restrictions the TPD will impose. We will continue to update you as we obtain more clarity.
Maximum size of 10ml for e-liquid bottles
This is clearly going to be a major problem for all sorts of reasons, not least because it makes it a much more expensive and inconvenient way to purchase and store e-liquid since it’s customary for many vapers to use 5 or 10ml or even more, per day. It’s completely at odds with regulators’ desire to reduce waste and promote a green agenda in every other area of our lives.
Some commentators have indicated they suspect this size limit is so the products can be very easily taxed per bottle at a later date although the regulators state that this limit was set with user safety in mind, to reduce the possibility of serious effect should someone accidentally or purposefully drink a bottle of e-liquid. We find it strange that the same type of restriction has not been applied to other readily available products that could be equally or much more dangerous than e-liquid if ingested orally. No-one’s ever seen fit to limit purchases of bleach to a thimbleful or limit the maximum bottle size for alcoholic spirits to the equivalent of a couple of units worth. It’s even more nonsensical when you consider that this very same TPD legislation has done the exact opposite of this restriction for cigarettes, in banning their sale in packs of fewer than 20 cigarettes to make them less appealing and less affordable to children.
This maximum bottle size issue is further compounded by the fact that buy-one-get-one-free and some other types of price promotion offers will be banned although it’s not clear whether the restrictions on price promotions will be imposed immediately or at the end of the sell-through period3.
You will no longer be able to legally purchase nicotine containing liquid in bottles larger than 10ml in any EU member state once the sell-through period3 is over.
Maximum strength of 20mg/ml
Research studies4 have found that vaping is actually less effective at delivering nicotine than smoking, and vapers ingest less nicotine from vaping than smoking. Many indicators (and our own experience) tell us that a higher strength liquid is often needed by some users in their first attempts to make a complete switch away from smoking. We believe most of our customers who currently vape 24mg/ml will be able to reduce to 18mg/ml or 20mg/ml, but there does remain a core group of vapers who require strengths outside the typical range to keep them from going back to smoking and those vapers will need to reduce the strength they use and consider hardware changes that could make a lower strength liquid more satisfying. This restriction is again related to regulators’ misplaced concerns about accidental poisonings based on out-dated information about the lethal dose of nicotine due to dubious self-experiments in the nineteenth century5. There will be no such restriction placed on the nicotine level in products that are manufactured by pharmaceutical companies and approved under medicines legislation as a licensed medicinal product for smoking cessation, however, these will almost all be cigalike type devices with single-use/disposable high strength cartridges.
You will longer be able to purchase non-pharmaceutical e-liquid higher than 20mg/ml nicotine (whether it’s pre-mixed, ready-to-vape or high strength liquid for DIY mixing) legally in any EU member state once the sell-through period3 is over.
Maximum 2ml size for cartridges and tanks
According to the EU directive, most of the current advanced tanks will be banned outright. However, according to the UK draft legislation, it appears that this restriction may only apply to tanks that contain e-liquid at time of sale. It’s also not entirely clear as to whether this includes rebuildables or replacement heads and coils but the most recent communications from the UK government appear to indicate it includes anything which could contain nicotine in the form of e-liquid which is intended to be vaporised and inhaled. Most of the tanks we currently stock and similar ones in the near future may not be allowed under the new rules, and those few that do meet the restrictions may not be saleable unless they have been tested and a full dossier provided to the MHRA 6 months in advance of them being placed on sale3 (or by November 2016 if they are already on sale).
If you currently prefer tanks larger than 2ml, you may not be able to purchase them legally in any EU member state once the sell-through period3 is over. We await some further clarification of exactly how this restriction will be applied in the real world.
Leakproof re-filling mechanism
The regulators have not yet fully defined what they mean here, and there’s been some discussion about whether or not the regulators might require some sort of e-liquid bottle docking system – which would obviously mean consumers will be “locked-in” to different types of proprietary refilling mechanisms and thus limited to which brand of liquids could be used with certain brands of tanks. It is hoped that the definition will be more related to the size and length of the tip on bottles, and the filling hole size on tanks.
We await further information on how/what this requirement will mean in the real world.
Electronic cigarettes must provide a consistent nicotine dose
Every user has a different vaping style and so everyone’s “puff duration” and pressure differs. Added to that, modern devices allow you to tailor the experience to your preferences with most advanced devices currently available featuring adjustability in wattage or voltage, and airflow. Defining “normal use” is therefore very challenging and indeed no interpretation or specification has been given. It looks like each manufacturer will be required to formulate their own dosage testing protocol and demonstrate that the amount of nicotine delivered is consistent and repeatable using this standard protocol.
We await further information on what this requirement will mean in the real world.
Product testing and notification
UK Manufacturers and importers of electronic cigarettes and refill containers will be required to perform very comprehensive, costly testing and submit a notification to the Medicines and Healthcare Products Regulatory Agency (MHRA) 6 months prior to placing a new or substantially altered product on the market. For the UK, the notification will need to be presented in a specific way which meets the MHRA’s adopted format, and shall include:
- A list of all ingredients contained in the liquid and contained in the vapour produced.
- Toxicological data for all those ingredients within both the liquid and the vapour.
- Information on nicotine doses and uptake for the product.
- Full description of all components of the product including where applicable, the opening and refill mechanism of the product.
- A description of the production process and a declaration of conformity with the requirements of the directive.
- Annual sales volume reporting across the notified products including geographical information and preferences of various consumer groups.
There will obviously be huge financial implications involved in the testing and notification process, which has been based on a pharmaceutical model despite the fact that these products are not pharmaceutical or healthcare products, and indeed retailers will still not be allowed to present them as such, or make any claims as to their effectiveness as a quit-smoking method. It would have been sensible for regulations to require testing and identification of known potentially harmful inhalant risks, but the regulators have seen fit to demand the entire recipe for all liquids, thus forcing manufacturers to disclose proprietary formulations which are essentially, trade secrets.
Food flavouring manufacturers aren’t currently required to divulge their entire recipes and can retain some commercial confidentiality to prevent formulations being stolen or copied by competitors. This makes it extremely difficult to identify compounds present because chemical analysis is performed by looking for specific ingredients. Without having knowledge of what one’s looking for, analysis is almost impossible. Even where the compounds are identified by the flavouring manufacturer, e-liquid manufacturers will still need to run chemical analysis on every single iteration of their product (that’s every strength and flavour separately) to identify and quantify all the compounds present, both in the liquid, and in the vapour. Cost estimates vary wildly, depending on who you speak to, but a very conservative estimate is a minimum of £5000 per iteration (e.g. a banana flavour e-liquid in 4 strengths = a minimum of £20,000 just for the testing and documentation for that one flavour liquid). There are also the initial notification and annual renewal fees to factor in as well as the huge administrative burden.
The resulting impact of these provisions is not yet known because e-liquid is currently available in the UK in thousands of flavour and strength combinations, all of which will be unsaleable unless they have been tested and notified. Very few manufacturers outside the EU will comply with these reporting requirements, so it will be up to the retailer to undertake the testing and notification and bear the cost. This is unlikely to happen in many cases, so many non-EU products will simply disappear from the shelves. EU manufacturers and retailers who can’t afford to test and prepare notification for hundreds of different products will likely close their business, or radically cull their ranges.
Once manufacturers do take note and make provisions to adhere to the new testing and notification requirements, they will not be able to place products on sale until 6 months after they make their product notification submission to the MHRA.
Expect to see a massive reduction in the e-liquid choices available on the EU market once the sell-through period3 is over and a delay on new products coming to market. Also expect e-liquid prices to increase.
New packaging requirements
The TPD requires that e-cigarettes and e-liquid bottles contain the following information on the outside of their packaging:
- A list of all ingredients contained in the product in descending order of the weight including the nicotine content of the product.
- An indication of the nicotine delivery per dose.
- The batch number.
- A recommendation to keep the product out of reach of children.
- The warning “This product contains nicotine which is a highly addictive substance. It is not recommended for use by non-smokers”.
The box must also contain an enclosure leaflet with:
- Instructions for use and storage of the product, including a reference that the product is not recommended for use by young people and non-smokers.
- Contra-indications.
- Warnings for specific risk groups.
- Possible adverse effects; Addictiveness and toxicity.
- Contact details of the manufacturer or importer and a legal or natural contact person within the EU.
- The warning “This product contains nicotine which is a highly addictive substance. It is not recommended for use by non-smokers”.
A fold-out information leaflet attached to the bottle may be acceptable in place of a box with leaflet enclosure.
Technically, there isn’t a definition for what a “dose” is as it relates to vaping because we ALL vape completely differently. Every vaper decides for themselves what strength they use, and how much they vape so this requirement could be slightly challenging. There are concerns over possible liability issues where the product packaging contains contraindications, and warnings for specific groups (whilst omitting other specific groups) and indeed this requirement is going to be a challenging one for all non-pharmaceutical companies due to the limitations of general product liability insurance. Most current retailers don’t even have sufficient product liability insurance now due to its cost, and it’s highly unlikely any small-medium retailer could afford sufficient insurance for products where health warning information is contained on their products.
Along with the reduction in choice, you can also expect to see a much higher cost for e-liquid, that has been tested, notified, and packaged in accordance with the regulations. Other repercussions such as product liability insurance issues are unclear.
References
- http://ec.europa.eu/health/tobacco/docs/dir_201440_en.pdf
- The TPD definitions for “electronic cigarettes” and “refill containers”:
‘electronic cigarette’ means a product that can be used for consumption of nicotine-containing vapour via a mouth piece, or any component of that product, including a cartridge, a tank and the device without cartridge or tank. Electronic cigarettes can be disposable or refillable by means of a refill container and a tank, or rechargeable with single use cartridges;
‘refill container’ means a receptacle that contains a nicotine-containing liquid, which can be used to refill an electronic cigarette; - Deadlines
20 May 2016 - New regulations apply for new products brought onto the market after this date.
20 Nov 2016 - Deadline for manufacturers and importers to submit data on ingredients and emissions for existing products; and manufacturers and importers of e-cigarettes to notify existing products on the market (if they intend to continue selling them after May 2017).
20 May 2017 - Date by which sell-through period for existing stock of non-compliant products purchased before November 20th 2016 ends. - http://www.ecigarette-research.com/web/index.php/research/2014/155-ecig-nicotine
?http://www.nature.com/articles/srep04133 - How much Nicotine kills a Human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century –Bernd Mayer – Archives of Toxicology January 2014, Volume 88, Issue 1, pp5-7
http://link.springer.com/article/10.1007%2Fs00204-013-1127-0/fulltext.html
Other resources
Tobacco and Related Products Regulations 2016
The EU Tobacco Products Directive
The counterfactual (Clive Bates)
Write to your MP. It's too late to change the TPD, but the implementation and enforcement strategy is not yet set in stone.
It's time to fight for vaping
You may have heard that the Medicines and Health Regulatory Agency (MHRA) intend to regulate e-cigs as medicinal products within the UK. This is coupled with the EU parliament heading in the same utterly unworkable direction. E-cigs are not medical devices, and nor were they ever intended to be. They are already very well regulated under the general product safety regulations framework, if only Trading Standards departments would properly clamp down on rogue traders who supply electrical goods that fall foul of British and European standards, and refuse to test their e-liquid or even label it properly and legally.
The MHRA have made all sorts of unjustifiable claims that regulating these devices as medicinal devices will make them safer and more effective. There hasn't been one single death attributed to e-cigarette usage, yet in the UK alone, 100,000 people die every year because of smoking.
You can attest to how safe and effective you've found vaping to be. In reality, the continuous pushing to shoe-horn these non-medicinal devices into medicines regulation is nothing about safety, harm-reduction or saving lives, and everything about those that make these decisions on your behalf receiving poor advice and making uneducated assumptions, and facing enormous pressure from both the pharmaceutical industry and the tobacco industry, both of whom are already losing revenue as more and more smokers make the switch to vaping.
If these proposals are forced through, vaping as we know it will die, all the wide variety of devices, atomisers, tanks, cartomisers, liquids and other amazingly wonderful products will be gone. In their place will be a small handful of ineffective, disposable type e-cigs from large pharmaceutical and tobacco companies. Think cig-a-like devices, with measured "doses" of liquid available in one or two flavours and useless for anyone but the very lightest occasional vaper.
We vapers are facing a war, fighting for our right to enjoy our e-cigs as a recreational alternative to traditional tobacco smoking. It needs all of us to do our bit.
What you NEED to do:
- Sign these two e-petitions here (only UK residents may sign the first one)
http://epetitions.direct.gov.uk/petitions/51572
http://www.petitiononline.com/fr33dom/petition.html
- Ask for an e-cigs save lives sticker when you place your next order, and display it on your car, shop window, or indeed anywhere it will be seen - request extras for family and friends too.
- Print out copies of the E-cigs Save Lives Flyer and distribute them as widely as you possibly can. Your workplace, your local shops, bars and restaurants, anywhere you can to help get the message across.
- Write to your MP and to your MEP, ask what they know about e-cigs, tell them what vaping means to you, and how limiting access to the myriad of different devices will impact you.
There's over one million vapers in the UK now, and it needs every single one of us to make our voice heard to protect our vaping way of life.
Our response to the MHRA announcement
For those customers who have seen and heard the news reports today regarding the MHRA's announcement about their intention to regulate electronic cigarettes as medicines. Many are emailing us to ask whether we will obtain a medicine licence or will we close.
The answer is neither!
Forcing our products to be licenced as medicinal devices is incorrect application of the medicines licensing framework, unethical and most likely illegal, given that traditional cigarettes are allowed to be sold freely to over 18s, and we are not making any claims as to a curative or medicinal effect or treatment for any disease. The MHRA decision will be challenged, we're not going anywhere, and will not be applying for a medicinal licence for any of our recreational products.
We do still urge all e-cig users to contact their local Members of Parliament and Members of the European Parliament to express their views about what restricting their access to these devices only in pre-packaged, "medically approved" format, from only pharmaceutically licenced vendors will mean to them and their new, improved way of life.