Home Creme de Vape Blog Posts on topic: tobacco products directive
Creme de Vape Blog
TPD Update
We understand there is still some confusion about the EU Tobacco Products directive (TPD) which came into law across the EU on May 2016. The UK implementation is known as the Tobacco and Related Products Regulations 2016 (TRPR). It contains various restrictions and regulatory burden all of which will affect our customers to varying degrees. We’re currently in the transition period, and we can sell existing stock without restriction. This period ends on 19th May 2017, after which all products sold must meet the new requirements of the TRPR. Liquids without nicotine are exempt from these requirements.
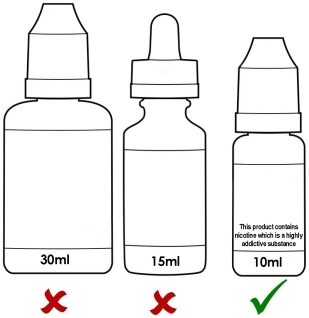
Maximum 10ml bottle size and maximum nicotine strength of 20mg/ml (2%).
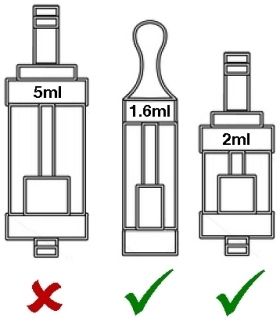
Maximum 2ml capacity for tanks, which must have a leak-free filling mechanism.
In addition to the size and strength restrictions, where a product contains nicotine, or could be used to contain nicotine the TRPR requires:
- A large warning about nicotine on the front and back of the packaging (even where the product is sold empty).
- Ingredients, composition and additional information and warnings to be on the outside of packaging and on enclosed leaflets.
- Products to be comprehensively tested (including their emissions during use) and the data notified to the governing body 6 months before products are placed on sale (or before November 20th 2016 for products already on the market).
- Sales of each notified product reported annually.
Some of the products you rely on may change to a compliant format, but many will not, and will become unavailable for sale to consumers in the UK and EU. The substantial costs involved in the testing and notification will lead to a further reduction in the variety of products on the market as most manufacturers will only be able to afford to test and submit their best sellers. Prices may increase slightly to cover the costs of the testing and notification, as well as the additional packaging.
We have been unable to import any non-compliant products since November 20th 2016, and so non-compliant products are beginning to sell out, and won’t be replenished. As you're browsing the website, you may notice a red box with the text "NOT PURCHASABLE IN THE EU AFTER MAY 19 This is your cue to buy now, whilst you still can, because you will not be able to purchase this product after this date. If you see this on a 30ml e-liquid, we recommend you browse the site to see if there is a 10ml compliant version available.
We advise you to ensure you have stocks of your favourite products on hand well before May and transition to compliant products as soon as they become available. We have been concentrating our efforts and working hard with our manufacturers to prepare compliant items over the last 6 months and although there are huge challenges to overcome, we are hopeful about the future of vaping post-2017.
For further information please see our earlier TPD blog post.
Last of the big tanks and more news
The TPD is due to go live this Friday 20th May and due to the new e-cigarette advertising restrictions we may be unable to send email newsletters. In future, we advise you to keep a close eye on our Coming Soon and Just Arrived pages regularly to see what's new.
Call to Action - Please write to your local MP
Last week we informed you about the Royal College of Physicians (RCP) report on electronic cigarette research, this week we are urging you to please write to your local MP asking them to support an early day motion calling for debate before the UK Parliment about the upcoming Tobacco Products Directive implementation on 20th of May this year.

Parliament UK
Early day motion 1441
E-CIGARETTES AND THE TOBACCO PRODUCTS DIRECTIVE
Session: 2015-16
Date tabled: 04.05.2016
Primary sponsor: Anne Main
That this House agrees with the Royal College of Physicians that it is crucial that e-cigarettes are priced as advantageously as possible in relation to tobacco.
Believes that the EU Tobacco Products Directive would significantly inhibit the development and use of harm-reduction products by smokers and cost lives.
Further agrees with Public Health England that e-cigarettes are around 95 per cent less harmful than smoking, and that nearly half the population does not realise that e-cigarettes are much less harmful than smoking.
Further believes that restricting advertising will have the perverse effect of reducing the rate at which cigarette use is declining.
Notes that the total cost of smoking to society, including healthcare, social care, lost productivity, litter and fires, was conservatively estimated by Action on Smoking and Health to be around £14 billion per year.
And calls on the Government to exclude e-cigarettes and other harm-reduction products from the Tobacco Products Directive.
You can write to your local MP using this website: https://www.writetothem.com/ Please be polite and explain why you feel the TPD will impact your choices for vapour products, and how e-cigs have changed your life. You need to be sure to urge your MP to support the "Early Day Motion 1441"
If you have not read the Royal College of Physicians' report and shared it with your friends and family members, please do so. The report highlights what we as vapers have known all along, here are just a few of the key points of the full report.
- Smoking is the biggest avoidable cause of death and disability, and social inequality in health, in the UK.
- People smoke because they are addicted to nicotine, but are harmed by other constituents of tobacco smoke.
- E-cigarettes are proving much more popular than NRT as a substitute and competitor for tobacco cigarettes.
- There are concerns that e-cigarettes will “renormalise” smoking, but to date, there is no evidence of this occurring to any significant degree in the UK.
- In the interests of public health it is important to promote the use of e-cigarettes, NRT and other non-tobacco nicotine products as widely as possible.
- There is a need for regulation to reduce adverse effects of e-cig use but the regulation should not be allowed to significantly inhibit the development and use of harm-reduction products.
It is excellent news that this comprehensive report fully recognises the importance of e-cigs as harm-reduction, however it may have come too late to prevent the damage that will be caused by the Tobacco Products directive coming into force next month, which will significantly inhibit the development and availability of these products. Be sure you have also read our blog post about the TPD (Tobacco Products Directive).
The future of vaping changes in May 2016
You may have heard rumours about upcoming regulations which will affect the availability of many of the vaping products you currently enjoy.
We’ve been hesitant to communicate much of this information directly to our customers thus far, because the situation has not been clear enough to be able to properly inform you about exactly what will happen. Information directly from UK regulators has slowly started to come through but there’s still grey areas and ambiguity in the information released so far, so it’s still quite difficult for companies to plan properly or advise their customers on what the future holds.
Having said that, we know a large proportion of our customers still don’t know anything about these looming changes and we felt it was time to give you a run-down of what we know so far as well as comment on what we don’t yet know. Apologies that this is quite a long post, but it’s a complex topic, and requires as much explanation as we can provide to ensure you are as well-informed as you possibly can be.
On May 20th 2016 The EU Directive 2014/40/EU1 also known as The Tobacco Products Directive (TPD) comes into force. This Directive changes how tobacco and related products are manufactured, marketed and sold within the EU and contains some very restrictive measures relating to vaping products2.
Many feel (us included) that the restrictions relating to electronic cigarettes didn’t belong within a directive relating to tobacco products at all, and that regulatory provisions for these innovative, and life-changing products belong in separate, more carefully considered legislation, which could have ensured vapers continued to have access to the products they need and the right to choose a much safer alternative to smoking. However, the directive as it is was pushed through to further regulate cigarettes, tobacco products, and vaping products, all in one go.
As a result of this legislation, many of the products we know and love will disappear and the products currently available on the market will become very limited. The rate of innovation will also slow due to the financial burdens and delay involved in bringing new products to market, compounded by the 6 month delay that’s been imposed for newly notified products. The regulations do however allow a sell-through period3 for the sale of old stock until May 2017.
Unfortunately, there’s still a LOT up in the air due to the regulators’ failure to unambiguously define what’s included and what isn’t as well as the fact they have repeatedly contradicted themselves on various documents we’ve seen. This uncertainty means that much misinformation is out there, and manufacturers and retailers are not yet able to state exactly what the position will be, even though the restrictions will take effect in just a few months’ time.
We’ve chosen to focus here on those elements of the legislation that will be most restrictive for existing vapers, however there are various other provisions that will dramatically affect vaping. One that is of great concern to those of us who are keen to spread the message of vaping to as many smokers as possible is the ban on all forms of advertising and promotional activity, which may include online discussion forums and specialist vaping broadcast channels as well as traditional print and TV/radio media. Please rest assured that we’re doing our absolute best to navigate our way through the legislation, and working with our manufacturers to ensure they are also up to speed, and capable of producing compliant products. However, these changes ARE coming, and all current and future vapers will be radically affected.
Read on for detailed information on some of the restrictions the TPD will impose. We will continue to update you as we obtain more clarity.
Maximum size of 10ml for e-liquid bottles
This is clearly going to be a major problem for all sorts of reasons, not least because it makes it a much more expensive and inconvenient way to purchase and store e-liquid since it’s customary for many vapers to use 5 or 10ml or even more, per day. It’s completely at odds with regulators’ desire to reduce waste and promote a green agenda in every other area of our lives.
Some commentators have indicated they suspect this size limit is so the products can be very easily taxed per bottle at a later date although the regulators state that this limit was set with user safety in mind, to reduce the possibility of serious effect should someone accidentally or purposefully drink a bottle of e-liquid. We find it strange that the same type of restriction has not been applied to other readily available products that could be equally or much more dangerous than e-liquid if ingested orally. No-one’s ever seen fit to limit purchases of bleach to a thimbleful or limit the maximum bottle size for alcoholic spirits to the equivalent of a couple of units worth. It’s even more nonsensical when you consider that this very same TPD legislation has done the exact opposite of this restriction for cigarettes, in banning their sale in packs of fewer than 20 cigarettes to make them less appealing and less affordable to children.
This maximum bottle size issue is further compounded by the fact that buy-one-get-one-free and some other types of price promotion offers will be banned although it’s not clear whether the restrictions on price promotions will be imposed immediately or at the end of the sell-through period3.
You will no longer be able to legally purchase nicotine containing liquid in bottles larger than 10ml in any EU member state once the sell-through period3 is over.
Maximum strength of 20mg/ml
Research studies4 have found that vaping is actually less effective at delivering nicotine than smoking, and vapers ingest less nicotine from vaping than smoking. Many indicators (and our own experience) tell us that a higher strength liquid is often needed by some users in their first attempts to make a complete switch away from smoking. We believe most of our customers who currently vape 24mg/ml will be able to reduce to 18mg/ml or 20mg/ml, but there does remain a core group of vapers who require strengths outside the typical range to keep them from going back to smoking and those vapers will need to reduce the strength they use and consider hardware changes that could make a lower strength liquid more satisfying. This restriction is again related to regulators’ misplaced concerns about accidental poisonings based on out-dated information about the lethal dose of nicotine due to dubious self-experiments in the nineteenth century5. There will be no such restriction placed on the nicotine level in products that are manufactured by pharmaceutical companies and approved under medicines legislation as a licensed medicinal product for smoking cessation, however, these will almost all be cigalike type devices with single-use/disposable high strength cartridges.
You will longer be able to purchase non-pharmaceutical e-liquid higher than 20mg/ml nicotine (whether it’s pre-mixed, ready-to-vape or high strength liquid for DIY mixing) legally in any EU member state once the sell-through period3 is over.
Maximum 2ml size for cartridges and tanks
According to the EU directive, most of the current advanced tanks will be banned outright. However, according to the UK draft legislation, it appears that this restriction may only apply to tanks that contain e-liquid at time of sale. It’s also not entirely clear as to whether this includes rebuildables or replacement heads and coils but the most recent communications from the UK government appear to indicate it includes anything which could contain nicotine in the form of e-liquid which is intended to be vaporised and inhaled. Most of the tanks we currently stock and similar ones in the near future may not be allowed under the new rules, and those few that do meet the restrictions may not be saleable unless they have been tested and a full dossier provided to the MHRA 6 months in advance of them being placed on sale3 (or by November 2016 if they are already on sale).
If you currently prefer tanks larger than 2ml, you may not be able to purchase them legally in any EU member state once the sell-through period3 is over. We await some further clarification of exactly how this restriction will be applied in the real world.
Leakproof re-filling mechanism
The regulators have not yet fully defined what they mean here, and there’s been some discussion about whether or not the regulators might require some sort of e-liquid bottle docking system – which would obviously mean consumers will be “locked-in” to different types of proprietary refilling mechanisms and thus limited to which brand of liquids could be used with certain brands of tanks. It is hoped that the definition will be more related to the size and length of the tip on bottles, and the filling hole size on tanks.
We await further information on how/what this requirement will mean in the real world.
Electronic cigarettes must provide a consistent nicotine dose
Every user has a different vaping style and so everyone’s “puff duration” and pressure differs. Added to that, modern devices allow you to tailor the experience to your preferences with most advanced devices currently available featuring adjustability in wattage or voltage, and airflow. Defining “normal use” is therefore very challenging and indeed no interpretation or specification has been given. It looks like each manufacturer will be required to formulate their own dosage testing protocol and demonstrate that the amount of nicotine delivered is consistent and repeatable using this standard protocol.
We await further information on what this requirement will mean in the real world.
Product testing and notification
UK Manufacturers and importers of electronic cigarettes and refill containers will be required to perform very comprehensive, costly testing and submit a notification to the Medicines and Healthcare Products Regulatory Agency (MHRA) 6 months prior to placing a new or substantially altered product on the market. For the UK, the notification will need to be presented in a specific way which meets the MHRA’s adopted format, and shall include:
- A list of all ingredients contained in the liquid and contained in the vapour produced.
- Toxicological data for all those ingredients within both the liquid and the vapour.
- Information on nicotine doses and uptake for the product.
- Full description of all components of the product including where applicable, the opening and refill mechanism of the product.
- A description of the production process and a declaration of conformity with the requirements of the directive.
- Annual sales volume reporting across the notified products including geographical information and preferences of various consumer groups.
There will obviously be huge financial implications involved in the testing and notification process, which has been based on a pharmaceutical model despite the fact that these products are not pharmaceutical or healthcare products, and indeed retailers will still not be allowed to present them as such, or make any claims as to their effectiveness as a quit-smoking method. It would have been sensible for regulations to require testing and identification of known potentially harmful inhalant risks, but the regulators have seen fit to demand the entire recipe for all liquids, thus forcing manufacturers to disclose proprietary formulations which are essentially, trade secrets.
Food flavouring manufacturers aren’t currently required to divulge their entire recipes and can retain some commercial confidentiality to prevent formulations being stolen or copied by competitors. This makes it extremely difficult to identify compounds present because chemical analysis is performed by looking for specific ingredients. Without having knowledge of what one’s looking for, analysis is almost impossible. Even where the compounds are identified by the flavouring manufacturer, e-liquid manufacturers will still need to run chemical analysis on every single iteration of their product (that’s every strength and flavour separately) to identify and quantify all the compounds present, both in the liquid, and in the vapour. Cost estimates vary wildly, depending on who you speak to, but a very conservative estimate is a minimum of £5000 per iteration (e.g. a banana flavour e-liquid in 4 strengths = a minimum of £20,000 just for the testing and documentation for that one flavour liquid). There are also the initial notification and annual renewal fees to factor in as well as the huge administrative burden.
The resulting impact of these provisions is not yet known because e-liquid is currently available in the UK in thousands of flavour and strength combinations, all of which will be unsaleable unless they have been tested and notified. Very few manufacturers outside the EU will comply with these reporting requirements, so it will be up to the retailer to undertake the testing and notification and bear the cost. This is unlikely to happen in many cases, so many non-EU products will simply disappear from the shelves. EU manufacturers and retailers who can’t afford to test and prepare notification for hundreds of different products will likely close their business, or radically cull their ranges.
Once manufacturers do take note and make provisions to adhere to the new testing and notification requirements, they will not be able to place products on sale until 6 months after they make their product notification submission to the MHRA.
Expect to see a massive reduction in the e-liquid choices available on the EU market once the sell-through period3 is over and a delay on new products coming to market. Also expect e-liquid prices to increase.
New packaging requirements
The TPD requires that e-cigarettes and e-liquid bottles contain the following information on the outside of their packaging:
- A list of all ingredients contained in the product in descending order of the weight including the nicotine content of the product.
- An indication of the nicotine delivery per dose.
- The batch number.
- A recommendation to keep the product out of reach of children.
- The warning “This product contains nicotine which is a highly addictive substance. It is not recommended for use by non-smokers”.
The box must also contain an enclosure leaflet with:
- Instructions for use and storage of the product, including a reference that the product is not recommended for use by young people and non-smokers.
- Contra-indications.
- Warnings for specific risk groups.
- Possible adverse effects; Addictiveness and toxicity.
- Contact details of the manufacturer or importer and a legal or natural contact person within the EU.
- The warning “This product contains nicotine which is a highly addictive substance. It is not recommended for use by non-smokers”.
A fold-out information leaflet attached to the bottle may be acceptable in place of a box with leaflet enclosure.
Technically, there isn’t a definition for what a “dose” is as it relates to vaping because we ALL vape completely differently. Every vaper decides for themselves what strength they use, and how much they vape so this requirement could be slightly challenging. There are concerns over possible liability issues where the product packaging contains contraindications, and warnings for specific groups (whilst omitting other specific groups) and indeed this requirement is going to be a challenging one for all non-pharmaceutical companies due to the limitations of general product liability insurance. Most current retailers don’t even have sufficient product liability insurance now due to its cost, and it’s highly unlikely any small-medium retailer could afford sufficient insurance for products where health warning information is contained on their products.
Along with the reduction in choice, you can also expect to see a much higher cost for e-liquid, that has been tested, notified, and packaged in accordance with the regulations. Other repercussions such as product liability insurance issues are unclear.
References
- http://ec.europa.eu/health/tobacco/docs/dir_201440_en.pdf
- The TPD definitions for “electronic cigarettes” and “refill containers”:
‘electronic cigarette’ means a product that can be used for consumption of nicotine-containing vapour via a mouth piece, or any component of that product, including a cartridge, a tank and the device without cartridge or tank. Electronic cigarettes can be disposable or refillable by means of a refill container and a tank, or rechargeable with single use cartridges;
‘refill container’ means a receptacle that contains a nicotine-containing liquid, which can be used to refill an electronic cigarette; - Deadlines
20 May 2016 - New regulations apply for new products brought onto the market after this date.
20 Nov 2016 - Deadline for manufacturers and importers to submit data on ingredients and emissions for existing products; and manufacturers and importers of e-cigarettes to notify existing products on the market (if they intend to continue selling them after May 2017).
20 May 2017 - Date by which sell-through period for existing stock of non-compliant products purchased before November 20th 2016 ends. - http://www.ecigarette-research.com/web/index.php/research/2014/155-ecig-nicotine
?http://www.nature.com/articles/srep04133 - How much Nicotine kills a Human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century –Bernd Mayer – Archives of Toxicology January 2014, Volume 88, Issue 1, pp5-7
http://link.springer.com/article/10.1007%2Fs00204-013-1127-0/fulltext.html
Other resources
Tobacco and Related Products Regulations 2016
The EU Tobacco Products Directive
The counterfactual (Clive Bates)
Write to your MP. It's too late to change the TPD, but the implementation and enforcement strategy is not yet set in stone.
Call to arms - why ALL vapers MUST act now!
Would you be affected if you could no longer easily buy your vaping supplies? What about if all you could get was e-liquid containing a paltry 4mg/0.4% nicotine? How would you feel about all the smokers who never got to experience the new way of life that you are currently enjoying? Upset? Depressed? Enraged?
Yep, us too, and here's your chance to try to do something about it. Please don't just think it's a lost battle, it is far from it, if YOU take the time to make SURE you have your say...
This article has been reproduced in its entirety from Clive Bate's blog because it gives you all the information you need to know and how to help do something about it to protect our vaping way of life.
EU draft Tobacco Products Directive: who to write to and what to say (a short guide) by Clive Bates
1. What has happened?
On 19th December 2012, the EU produced a a proposal for new laws controlling tobacco and nicotine products like e-cigarettes. The proposal includes a justification for the measures with draft legal text and comes with supporting documentation. It covers a wide range of issues, including: labelling and warnings on cigarette packs; branding restrictions; control of flavours and additives; tracking and tracing to prevent smuggling and counterfeiting; and measures that apply to smokeless tobacco products and nicotine-containing products like e-cigarettes.
The proposed directive contains measures that make it harder or impossible for smokers to switch from cigarettes to much less dangerous nicotine products – an approach that will cause much more death and disease than it prevents. If you smoke, use e-cigarettes, or if have friends or relatives that smoke, or if you are concerned about the health damage from smoking, then this directive matters to you.
If you want to write to your MP or MEPs, the main information you need together with my advice is set out below. More detailed briefing on the directive is available here and on influencing the scrutiny process is here. A good political article on the directive here. A good background video on tobacco harm reduction here.
2. Why do you need to act?
The European Union has proposed legislation that would ban, or effectively ban, nicotine products that people can (and do) use as much less dangerous alternatives to smoking cigarettes – for example, smokeless tobacco, e-cigarettes, vapour devices, or other novel ways of taking nicotine that don’t involve burning tobacco. Although the risk is reduced by 95-99% if there is no smoke, Europe still thinks it is better to ban these products even though they are a potential life-saver for people who can’t or don’t want to give up nicotine. If you use these products yourself, they are restricting your options and adding to your health risks. Background information on ‘tobacco harm reduction’ here
3. What are they doing?
The proposed new law (an EU directive) does three main bad things:
1. Bans the safest tobacco products. It bans the least hazardous form of tobacco known to mankind – snus – whilst allowing cigarettes to be widely sold. Snus (or oral tobacco) is much less dangerous than cigarettes, and widely used in Sweden, where it is the main reason why Sweden has much lower rates of cancer and other smoking-related disease than anywhere else in Europe.
Why ban these products when they have been so successful at reducing harm in Sweden?
2. Treats e-cigarettes as though they are medicines – effectively banning or marginalising them. It places most non-tobacco nicotine products, like e-cigarettes, under the highly restrictive regulation regime used for medicinal products. This requires the manufacturers or distributors to justify them for their therapeutic effect and to demonstrate that benefits outweigh the risks – even though that may be obvious to most people, it is potentially difficult to do it to scientific standards. In fact these are really consumer products chosen by consumers as an alternative to smoking the most unhealthy forms of nicotine rather than medicines – and should be judged as alternatives to cigarettes. Depending on the attitude of medicines regulators this type of regulation could have the several negative effects. It could:
- amount to an effective ban if regulators demand impossibly high standards of proof or
- take these products off the market in 2013 as there is no ‘transition’ period to allow manufacturers to apply for and get the necessary authorisation and it would be illegal to sell them as soon as the directive comes into force, which could be as soon as 2013
- take these products off the market for many years as most or all manufacturers will struggle to get the necessary ‘marketing authorisation’ from regulators, who may all disagree with each other around Europe
- apply restrictions that make these products unattractive to smokers through packaging requirements, marketing restrictions, bans on flavours, technical limitations imposed;
- greatly close down competition, limit innovation, raise costs leaving the market to big players, such as tobacco or pharma companies, that can cope with the huge burdens that comes with medicines regulation.
The directive treats e-cigarettes below a certain threshold as consumer products. The very weakest form of e-cigarettes (with liquids below threshold of nicotine density 4mg/ml) might escape medicines regulation. But these are extremely weak in e-cigarette terms, and not regarded as adequate substitutes for conventional cigarettes and unlikely to do much to help people switch from smoking. More on this in my briefing on the directive.
Why would governments make it harder to put these products on the market than the much more dangerous products they are designed to replace or compete with? Read novelist Lionel Shriver (We need to talk about Kevin) on Puritans and the powerful – and tobacco smokers – can’t take the fact that electronic cigarettes are harmless and enjoyable
3. Prevents any claim that one tobacco product is less harmful than another. The trouble is that the truth is that smokeless tobacco products may be many times less harmful than cigarettes, perhaps 10-1000 times less harmful. So what looks like an attempt to stop false or excessive claims, is actually going to do real harm:
- It denies consumers the most relevant information about lower risk tobacco products – information they could use to reduce their own risk and protect their health This is misleading by omitting the most important information.
- Why should a manufacturer bother to make or market these products or invest in innovation if they can’t say the one (truthful) thing that makes them valuable as alternatives to cigarettes? All this does is reinforce the market for the most harmful tobacco products by shielding them from competition from less harmful forms.
This makes a law out of misleading consumers – who benefits from it?
4. Does it matter?
Yes it does – the health of real people is at stake. Smoking already kills 700,000 and costs €25 billion in health care costs in Europe annually (about 100,000 and £3.7 billion for the UK) [source]. Quit rates remain stubbornly low despite years of effort and drug development – and 28% of European adults still smoke (about 21% in the UK) despite almost universal knowledge of the dangers. Most smokers say they would like to quit and most say they wish they had never started. Some like a nicotine hit and some of the ritual that goes with smoking, but we know that if safer alternatives can be found people will use them them. There is a grave danger that people denied much safer alternatives will either lapse back to smoking or never be able to try these ways of giving up smoking. I have never seen a directive where the evidence so clearly points to it causing more death and disease – it is reckless, irresponsible, unscientific and unethical.
5. What to do: write to your MP and MEPs
Your Member of Parliament (MP) represents you in the UK, and several Members of the European Parliament represent you in matters to do with the European Parliament. Both MPs and MEPs have a role to play on the tobacco directive, so it is best to to write to both. Your MP can approach UK government ministers and ask them to influence the directive as it passes through the European Council (comprised of ministers of the member states). Your MEPs can influence the European Parliament scrutiny of the directive, propose amendments and influence the stance taken by political groupings in the European Parliament. If they are members of the Environment, Public Health and Food Safety Committee, they will be involved more directly in scrutinising the directive.
The simplest way to do this is to access www.writetothem.com. This is an excellent service: you enter your postcode; it works out who your MP and MEPs are (you will have several MEPs) then sets up e-mails for you to send them. You enter your own text and address details and then follow the procedure on the site and it will send your letter. Once you have drawn up a good letter that covers most of the points you want to get across you can use it for lots of different purposes – customising and personalising for each if you want to make an impact.
For non-UK readers. For non-UK readers, I have less information – but all MEPs can be located here – and information on how different national parliaments scrutinise EU legislation can be found here. Your can follow many of the tips here and tailor for your national situation.
Some Tips on Writing to MPs and MEPs
- Decent. Always be polite and dignified, don't make accusations or pre-judge their motives - most representatives want to do a good job for you.
- Engaging. Work on the basis that the MP or MEP is open-minded but might need some persuading. Don't dismiss other views, tackle them.
- Authentic. Write your own views in your own words - MPs and MEPs want to hear genuine heartfelt views, and not standard letters or borrowed text.
- Natural. Don't feel you need to use formal or legal language - it is their job to understand you, not your job to understand the technicalities of EU legislation.
- Concise. Concentrate on the things that really matter to you and stay focused - if you are writing about e-cigarettes, don’t dilute your message with views on other issues or even other aspects of the directive unless they really matter to you. Keep it short (max 2 pages or 800 words) and to the point.
- Personalised. Even though the web site allows you to send a single letter to all your MEPs in one go, I would advise emailing each individually. You an use the same basic text with each, but a little bit of a personal touch goes a long way.
- Relevant. Only write to your own MP or MEPs.
If you want to write on proper paper and post a letter, you can use the www.writetothem.com site to find out their names and then post a letter (stamp to Brussels is 87p for a letter). The addresses are:
Their Name MP
House of Commons
London SW1A 0AA
Their Name MEP
European Parliament
Rue Wiertz
B-1047 Brussels
Belgium
6. What to say
It is important that you write in your own words, based on your own experience and express your own views. I must stress this – authenticity really matters.
6.1 A good letter to an MP or MEP might have the following main components:
- About you and your experience – eg. have you tried to quit smoking? What effect has vaping had on you? What experience have you had of e-cigarettes? What you think of the threshold e-liquids?
- Why you think what is proposed will be bad, especially if it is bad for you personally.
- What you think should be done, and what you would like them personally to do
- Questions that make sure you get a response: ask questions, ask for a reply and/or ask for a meeting
6.2 Reasons why the directive might be bad
You don’t need to use any or all of these, but they might help you construct a letter. Remember to personalise these to reflect your own situation where possible.
- The proposed directive seems to deny or obstruct smokers options to quit cigarettes by switching to nicotine or tobacco products that are much less risky. This is very risky and irresponsible, and will probably cause more death and disease.
- It looks like it is designed to tie up e-cigarettes and their makers in medical red tape, which could amount to banning them by the back door – and it makes no sense to ban them whilst leaving real cigarettes on the market.
- Even if medicines regulation doesn’t stop these products getting to the market, it may place restrictions on them making them less attractive, more expensive and less innovative – for example by banning flavours, making the packing look like medicines, and strictly limiting advertising and marketing. We don’t really know how medicines regulators will treat these these products.
- It could mean e-cigarettes are taken off the market while the makers apply for permission – there should be 2-3 years transition to give existing products time to comply with the directive.
- It is wrong to pretend that all tobacco and nicotine products are the same – smokers should have true and relevant information about risks so that they can make informed choices.
6.3 What should be done?
These are a few suggestions from me.. please pick ones that matter to you, add your own views and use your own words.
- There should be no ban on oral tobacco (snus) – instead all smokeless tobacco should be regulated to reduce any toxic substances in the tobacco. This product is much safer than cigarettes and is a viable substitute for smoking. Smokers should not be denied this option.
- E-cigarettes should remain regulated for what they are – consumer products, placed on the market as alternatives to cigarettes. The appropriate regulatory regime is that used more generally for products – ’General Products Safety Regulation’, which is governed by an EU Directive and UK regulations.
- Only where an e-cigarette maker wants to make a health or ‘therapeutic claim’ should medicines regulation apply – otherwise treat it like a consumer product. If they don’t make a therapeutic claim, how can they provide evidence for it? It makes no sense to apply really tough regulation to these products and much weaker regulation to cigarettes when they are just competing to be alternatives to appeal to consumers.
- If the EU is determined to press ahead with applying medical regulation there should be three year transitional period to allow the makers to submit applications to sell these products and to ensure they don’t disappear from the markets overnight when the directive enters into force, thus forcing many users back to smoking.
- That products like e-cigarettes should remain on the European market – otherwise there will just be a flood of internet sales and all the business will be done with traders outside the EU.
- The European Union should find ways to encourage smokers to switch to e-cigarettes or smokeless tobacco, not ban or marginalise these products through regulation.
6.4 What you could ask your MP or MEPs to do…
Write to your MP and MEPs – you need slightly different letters because they have different roles and can do different things:
- Ask your MP and MEPs to reply to you… ask them to give their views on the parts of the proposed directive that deal with smokeless tobacco products and nicotine containing products.
- If you are an e-cigarette user, ask your MP and MEPs to give an undertaking that they will not support an EU directive that removes most or all e-cigarettes from the EU market, and point out this is important for your own health.
- Ask to meet your MP and MEPs, and tell them you would like them to understand why this matters to you by explaining it in person.
- Ask your MP to raise your concerns with the Secretary of State for Health (Rt Hon Jeremy Hunt MP) and Secretary of State for Business, Innovation and Skills (Rt Hon Dr Vince Cable MP). Ask your MP to ask them to press for amendment of the directive as it will harm health and works against the EU single market.
- Ask your MEP to raise your concerns with the Commission and to speak out in the debate in the European Parliament.
- Advanced! Ask your MEP to contact the relevant MEPs on the ENVI committee and to make their views known. If your MEP is on the ENVI committee, ask them to raise your concerns in the committee sessions that scrutinise the directive. This is a list of members of the ENVI committee or you can consult my more detailed guide on how this will all work.
7. What else?
If you do write to a representative, then you could leave a copy of your letter here in the comments as an inspiration for others.
If you have questions about the directive or disagreements with Clive's interpretation or advice, please comment on his blog and he’ll respond, and change as necessary.
Here's Clive talking to Dave Dorn on VaperTrails TV
This article has been reproduced in its entirety from Clive Bate's blog because it gives you all the information you need to know and how to help do something about it to protect our vaping way of life.